|
|
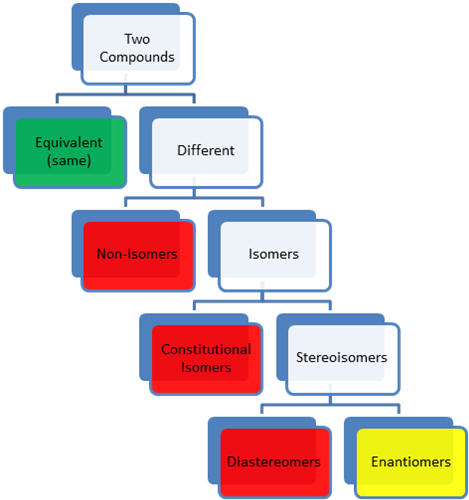
- two compounds
- two structures can have different conformations and still be
considered to be equivalent because they can be rotated along sigma
bonds to be equivalent (same) or super-imposable
- different compounds
- non-isomeric compounds have different atoms or number of atoms
- non-isomeric compounds have different properties
- isomers are different compounds that have the same molecular
formula
- constitutional or structural isomers are connectivity
isomers (different base name)-same atoms different connections
- constitutional isomers have different properties
- stereoisomers have the same connectivity (same base name)
but different three-dimensional arrangement of atoms
- diastereomers are not mirror images of each other
- diastereomers can be chiral or achiral
- examples
-
cis vs. trans
-
E vs. Z
-
endo vs. exo
-
syn vs. anti
-
r vs. s
-
RR vs. SR
- diastereomers have different properties
- diasteromers have different physical properties
- diastereomers have different biological
properties
- enantiomers are non-super-imposable (different) mirror
images
- symmetric molecules do not have enantiomers because
they contain two mirror images so when viewed in a
mirror you see both mirror images again or
the original object again
-
point symmetry
-
axis and reflection symmetry
-
plane symmetry
- enantiomers are asymmetric (dissymmetric)
- asymmetric molecules are also called chiral like the
Greek for hand
- symmetric molecules are also called achiral
- an example of a chiral molecule is a molecule with
an odd number of chiral centers
- chiral centers are tetrahedral atoms with four
different (non-isomeric, constitution, diastereomers)
groups attached
- carbon
- nitrogen in
aziridines but otherwise
tunneling of non-bonding electrons leads to
inversion of configuration easily on nitrogen
- sulfur (sulfone) where non-bonding electrons
are given the lowest priority (Prilosec, Nexium)
- the configuration (R or S) of
chiral centers is assigned by the Cahn-Ingold-Prelog
rules
- the chiral center can be represented using
wedges (forward) and slashes (back)
- Fischer projections (bow ties on the
horizontal lines)
- Newman projections (circle is back carbon)
- Haworth projections (hexagon or pentagon
with vertical lines)
- tetrahedral atoms that have four different
groups but two of the groups are mirror images of
each other are not chiral centers, they are pseudo-chiral
centers, assigned with little r and s.
- tetrahedral atoms that have four different
groups that are two pairs of mirror images are
chiral centers
- chiral centers and pseudo chiral centers are
called stereogenic centers because interchanging the
position of two groups on the centers (also called
inversion of configuration) leads to a new
stereoisomer
- interchanging two groups on a chiral center
in a molecule with one chiral center leads to
enantiomers
- interchanging two groups on a chiral center
in a molecule with more than one chiral center
leads to diastereomers called epimers (one
exception)
- interchanging two groups on a pseudo-chiral
center leads to diastereomers
- molecules with an even number of chiral centers
(including zero) may be chiral or not
-
allenes are examples of molecules that are
chiral but do not have chiral centers
-
spiro compounds are examples of molecules that
are chiral but do not have chiral centers
- conformational isomers may also be chiral
-
biphenyls
-
binaphthyls
- symmetric molecules with chiral centers (R, S)
are called meso
- enantiomers have the same properties in symmetric
environments
- enantiomers have the same physical properties
- melting point, boiling point, solubility,
heat of combustion, IR, NMR, etc.
- enantiomers have different properties in asymmetric
environments
- enantiomers have different biological activity
because biomolecules are chiral
- you are what you eat (cereal box chemicals)
-
carbohydrates are mostly D
- alpha-amino acids (building blocks of
proteins, enzymes) are mostly L
- lipids
-
triglycerides
-
cholesterol
- smell
- carvone
- (-)-enantiomer =
(S)-2-methyl-5-(1-methylethenyl)cyclohex-2-en-1-one
=
mint
- (+)-enantiomer =
(R)-2-methyl-5-(1-methylethenyl)cyclohex-2-en-1-one
=
rye bread
- limonene
-
orange smell-(+)-(R)-1-methy-4-(pro-1-en-2-yl)cyclohex-1-ene
-
pine smell-(-)-(S)-1-methy-4-(pro-1-en-2-yl)cyclohex-1-ene
- citronellol
-
citronella candels-(+)-(R)-3,7-dimethyloct-8-en-1-ol
-
rose smell-(-)-(S)-3,7-dimethyloct-8-en-1-ol
- enantiomers can be differentiated by optical
rotation using polarizers in a polarimeter
- rotation = specific rotation x concentration
x path length
- specific rotation = rotation/(c x l)
- specific rotations may be over 360° as
observed with low concentrations of compound
- (+)-dextrorotatory enantiomer, old d
- (-)-levorotatory enantiomer, old l
- ([major enantiomer]-[minor enantiomer])/([major
enantiomer]+[minor enantiomer]) = enantiomeric
excess = e.e., assuming only enantiomers
- |observed specific rotation (assuming only
enantiomers)| = e.e. x rotation of pure (+)-enantiomer,
- e.e. = |observed specific rotation| /
rotation of pure (+)-enantiomer
- if you make [major enantiomer] + [minor
enantiomer] = 1, relative concentration
- e.e. = [major enantiomer]-[minor
enantiomer]
- e.e.+1 = 2 x [major enantiomer]
- [major enantiomer] = (e.e.+1)/2
- [minor enantiomer] = 1-(e.e.+1)/2 =
(1-e.e.)/2
- an equal mixture of enantiomers is called a racemic
mixture
- racemic mixtures have different properties than
those of pure enantiomers
-
D, L tartaric acid
(+)-(2R,3R)-2,3-dihydroxybutanedioic
acid
-
meso-tartaric acid
(2R,3S)-2,3-dihydroxybutanedioic
acid
-
racemic tartaric acid
- enantiomers can be separated or resolved by
forming diastereomers or diastereomeric interactions
- for example, racemic carboxylic acids can be
resolved by forming diastereomer salts with
chiral amines
- enantiomers can be separated using chiral
chromatography
- separation of enantiomers is big business
- the enatiomeric switch
- Prilosec goes to Nexium
- a molecule with n chiral centers
- can have up to 2n-# meso structures =
stereoisomers in its family or
- 2n - # meso structures -1 stereoisomers
- if a compound is chiral it has only one enantiomer
- if a compound is achiral it does not have an
enantiomer
- if a compound is chiral it can have up to 2n-2
diasteromers
|
|
 Stereochemistry
Stereochemistry