|
|
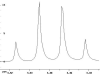
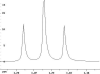
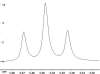
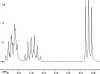
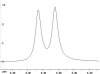
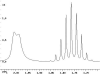
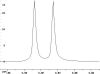
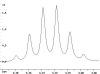
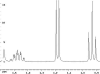
The following are 1H NMR spectra of isomers of C4H10O.
To determine the structure of each:
- Use the cyclomatic formula to determine how many rings plus
unsaturations this molecular formula corresponds to. Knowing of the
possibility of rings and unsaturations is a great start!
- Determine how many different types of protons appear in each spectrum.
Remember that equivalent or enantiotopic protons have the same chemical
shift where as diastereotopic and constitutional heterotopic protons should
have different chemical shifts. The chemical shift values may help you
determine what type of protons you have, e.g. a methyl at < 1 ppm, but
remember that anisotropy messes everything up.
- Measure the height of the integration curves with a ruler to estimate
the ratio of each type of proton.
- Multiply your ratios, e.g. 1:1:3, by the same number so that the sum
of each type equals 10.
- You may have to round up or down to get whole numbers because of
improper phasing.
- Assign the multiplicity or splitting of each proton signal to determine
how many hydrogen neighbors each set of protons has.
- Singlet means no neighbors.
- Doublet means one.
- Triplet means two.
- N equals N-1 neighbors.
-

-
-
-
-
|
|
 NMR
NMR NMR
NMR